About the performance of thermally conductive diamond
Time:
2024-12-05
With the advancement of science and technology, diamond is increasingly used as a super material in areas such as sound./Light/Electricity/Heat, etc.
DiamondAs the hardest known natural material, it has a wider bandgap, a broader range of light transmission, the lowest compressibility, and the highest thermal conductivity at room temperature, and it exhibits chemical inertness to most substances.It isparticularlysuitable for use in high-temperature, high-pressure, and high-frequency environments..
In terms of thermal properties, diamond is the material with the highest thermal conductivity in nature, reaching up to2000~2200 W/m·K,which is 4 times greater than silicon carbide (SiC), 13 times greater than silicon (Si), and 43 times greater than gallium arsenide (GaAs), and 4 to 5 times greater than copper and silver.Modern high-power electronic and optoelectronic devices (such as 5G applications, high-speed computing, or high-power semiconductor chips) generate a large amount of heat in a very small area, facing serious cooling problems. To achieve rapid cooling, high thermal conductivity materials are needed to create heat sinks or thermal coatings arranged at the heat-generating end (such as radiators, fans, heat sinks, etc.). Diamond, which has extremely high thermal conductivity, very low thermal expansion coefficient, and is an insulator at room temperature over a wide temperature range, has become the optimal choice.Diamond can meet the ultra-high requirements of thermal management applications in two main forms: one is diamond films, and the other is using diamond as a thermal conductive filler. Currently, thermally conductive diamond fillers are mainly applied in diamond-copper and diamond-aluminum metal matrix composites (MMC, Metal Matrix Diamond Composites) and thermal interface materials (TIM, Thermal Interface Materials). This discussion mainly focuses on two directions: diamond thermal conductive gel.Metal matrix diamond compositesDiamond, as a reinforcement phase, has an extremely high thermal conductivity (up to 600~2200 W/m·K at room temperature), which makes metal matrix diamond composites perform excellently in thermal conductivity. For example, the thermal conductivity of diamond/copper composites can reach up to 602 W/m·K when the diamond volume fraction is 35%. This high thermal conductivity makes it very suitable for applications requiring efficient heat dissipation, such as electronic packaging and high-power electronic devices.Moreover,the low thermal expansion coefficient of diamond (about 2.3×10-6 K-1) can effectively reduce the thermal expansion coefficient of the material when combined with a metal matrix (such as copper or aluminum). This characteristic helps to minimize dimensional changes in materials during temperature fluctuations, enhancing the stability and reliability of devices.
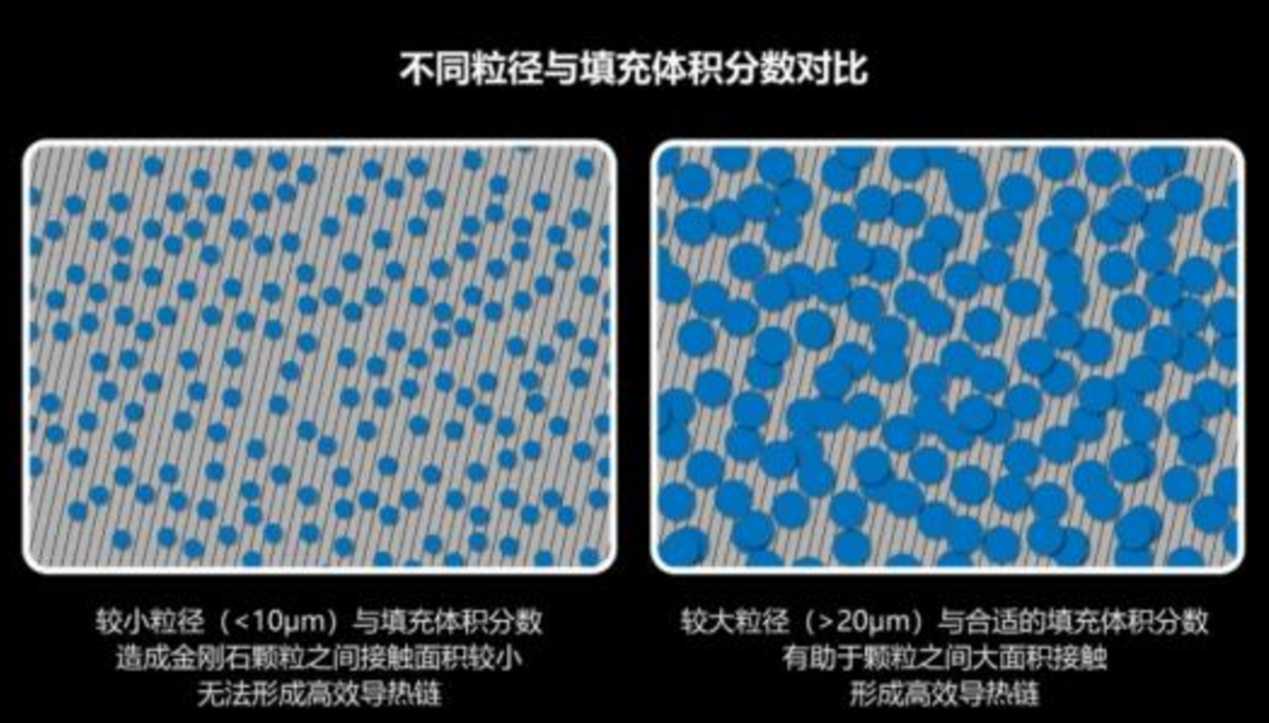
Diamond thermal conductive gelLike other thermal conductive gels, the performance of diamond thermal conductive gel largely depends on whether the preparation process is mature and excellent. Factors such as the particle size of the filler, filling volume fraction, and modification process all have a significant impact on the overall thermal conductivity of the gel.First, the particle size of diamond particles should not be too small (less than 10 microns), otherwise it will be difficult to form an effective thermal conduction chain.Secondly, the filling volume fraction of diamond particles should not be too large or too small. If it is too small, the contact area will also be small, making it difficult to form an effective thermal conduction chain; if it is too large, the gel will not be able to fully wet the surface of the diamond particles, leading to voids, which will also affect thermal conductivity.Finally, modification is also a necessary process for this type of thermal conductive gel filler. Otherwise, these surface-active particles are prone to agglomeration, making it difficult to achieve uniform dispersion in organic polymer resins, resulting in decreased gel performance.Why is diamond's thermal conductivity so high?,Generally, materials with the simplest chemical composition and molecular structure have the highest thermal conductivity values.In crystallography, the diamond structure is also known as the diamond cubic crystal structure, formed by carbon atoms bonded together through covalent bonds. Many of diamond's extreme properties are a direct result of the strength of the rigid structure formed by sp³ covalent bonds and the interaction of a small number of carbon atoms. Metals conduct heat through free electrons, and their high thermal conductivity is associated with high electrical conductivity. In contrast, heat conduction in diamond is solely accomplished by lattice vibrations (i.e., phonons). The extremely strong covalent bonds between diamond atoms give the rigid lattice a high vibration frequency, resulting in a Debye characteristic temperature as high as 2,220 K. Since most applications are far below the Debye temperature, phonon scattering is minimal, leading to very low thermal conduction resistance mediated by phonons. However, any lattice defects will cause phonon scattering, thereby reducing thermal conductivity, which is an inherent characteristic of all crystalline materials. Defects in diamond typically include point defects such as heavier ¹³C isotopes, nitrogen impurities, and vacancies, as well as extended defects such as stacking faults and dislocations, and 2D defects such as grain boundaries.
Other information (not for promotional materials, for learning purposes only):
Since diamond relies on phonons for heat transfer, generally, the lower the nitrogen content in diamond, the higher the thermal conductivity, and the more complete the crystal structure, the higher the thermal conductivity.For example, the IIa type with the purest and least impurities has a thermal conductivity as high as 26 watt/K-cm, which is 6 to 7 times faster than the fastest heat-conducting metals, silver and copper.而金刚石的低热膨胀系数(约2.3×10-6K-1)与金属基体(如铜、铝)结合后,能够有效降低材料的热膨胀系数。这种特性有助于减少材料在温度变化时的尺寸变化,提高设备的稳定性和可靠性。
金刚石导热凝胶
就像其他导热凝胶一样,金刚石导热凝胶的性能优劣,很大程度上取决于制备工艺是否成熟且优秀,如填料的粒径、填充体积分数、改性工艺等,都对凝胶整体的导热性能有着举足轻重的影响。
首先,金刚石颗粒的粒径不能过小(小于10微米),否则很难形成有效的导热链。
其次,金刚石颗粒的填充体积分数也不能过大或过小,过小的话接触面积也小,一样很难形成有效的导热链;过大的话凝胶无法充分浸润金刚石颗粒表面会造成空隙,同样影响热导率。
最后,改性也是这类导热凝胶填料的必经之路,否则这些表面活性高的粒子极容易团聚在一起,难以在有机高分子树脂中实现均匀分散,导致凝胶性能下降。
为什么金刚石的导热率这么高呢?
通常,化学成分和分子结构最简单的材料具有最高的热导率值。
在晶体学中,金刚石结构又称为金刚石立方晶体结构,由碳原子通过共价键结合形成。金刚石的许多极致属性都是形成刚性结构的sp³共价键强度和少量碳原子作用下的直接结果。金属通过自由电子传导热量,其高热传导性与高导电性相关联,相比之下,金刚石中的热量传导仅由晶格振动(即声子)完成。金刚石原子之间极强的共价键使刚性晶格具有高振动频率,因此其德拜特征温度高达2,220 K。由于大部分应用远低于德拜温度,声子散射较小,因此以声子为媒介的热传导阻力极小。但任何晶格缺陷都会产生声子散射,从而降低热传导性,这是所有晶体材料的固有特征。金刚石中的缺陷通常包括较重的ˡ³C同位素、氮杂质和空缺等点缺陷,堆垛层错和位错等扩展缺陷以及晶界等2D缺陷。
其他信息(不写道宣传册上,仅供学习):
由于金刚石依靠的是声子进行传热,一般情况下,金刚石中氮含量越低,热导率越高,晶型越完整,热导率越高——比如说最纯净、杂质最少的IIa型导热系数就高达26 watt/K-cm,比传热最快的金属银、铜还要快 6~7 倍。
In addition, the local variations in non-diamond carbon (NDC) content, grain size, or surface roughness may also affect the local measurement of thermal conductivity.Factors affecting the thermal conductivity of diamond/copper composites.
DiamondTheoretically, diamond/copper composites have excellent overall performance suitable for electronic packaging materials, but in practice, the actual thermal conductivity of diamond/Cu composites produced is relatively low, mainly due to the immature processing technology and complex preparation process of diamond/copper composites. Based on current research, the factors affecting the thermal conductivity of diamond/Cu composites can be summarized as follows.
1. The intrinsic thermal conductivity of the copper matrix.The lower the impurity content in the copper matrix, the higher the intrinsic thermal conductivity. For example, when the chromium content in the copper matrix reaches 0.1% (at/at), the thermal conductivity drops to 260 W·m-1·K-1. During the preparation of composites, different interface elements have different solubility when in contact with the copper matrix. On one hand, the dissolution of interface elements into the matrix leads to a decrease in the intrinsic thermal conductivity; on the other hand, the solid solution or compound formed at the interface after the dissolution of interface elements in the copper matrix is detrimental to heat transfer.
2. The intrinsic thermal conductivity, volume fraction, and particle size of diamond.
Generally, the lower the nitrogen content in diamond, the higher the thermal conductivity, and the more complete the crystal form, the higher the thermal conductivity. Therefore, diamonds with complete crystal forms and low nitrogen content should be selected as the reinforcement phase for composites. In addition, the diamond surface is easily transformed into a poorly conductive graphite-like phase under the influence of high temperature and catalytic elements, severely affecting the intrinsic thermal conductivity of diamond, thereby impacting the thermal conductivity of the composite.Theoretically, the higher the volume fraction of diamond, the higher the thermal conductivity of the composite. In fact, it depends on the preparation process. Using the infiltration method to prepare diamond composites with a volume fraction of 60-65% can achieve relatively high thermal conductivity. The particle size of diamond is also a factor affecting the thermal conductivity of the composite. Research has found that nanoscale diamonds tend to agglomerate, resulting in high porosity and low thermal conductivity in the prepared composites. It is generally believed that diamond composites with particle sizes of 100-500 μm can achieve higher thermal conductivity.
3. Interface thermal conductivity.
Interface thermal conductivity is an important criterion for evaluating whether the interface structure of composites is beneficial for improving thermal conductivity. Therefore, factors affecting interface thermal conductivity determine the thermal conductivity of the composite.
In addition to the intrinsic thermal conductivity of the matrix and reinforcement, the content and size of the reinforcement, the composite interface is a key factor determining the thermal conductivity enhancement effect of the reinforcement in a specific material system. For diamond/Cu composites, the thermal conductivities of Cu and diamond have theoretical limitations (generally not exceeding 400 and 2000 W/(m·K), respectively). Although both theoretical and experimental studies indicate that using high content and large particle size diamonds has significant advantages in improving the thermal conductivity of composites, it is unrealistic to infinitely increase the particle size of diamonds (generally not exceeding 400 μm) and volume content (generally not exceeding 70%), and it poses significant challenges to the material's molding densification, dimensional accuracy, surface roughness, surface gold plating treatment, and micro-stress distribution, severely restricting the yield and applicability of the products. Therefore, how to effectively reduce interface thermal resistance is key to achieving high thermal conductivity in diamond/Cu composites.For the preparation of composites, mutual wetting between components is a necessary prerequisite for compounding and is an important factor affecting the interface structure and bonding state. The non-wetting condition between diamond and Cu leads to very high interface thermal resistance. Therefore, it is crucial to conduct modification research on the interface between the two through various technical means. Currently, there are mainly two methods to improve the interface issue between diamond and Cu matrix:
1) Surface modification treatment of diamond.
For example, coating active elements such as Mo, Ti, W, and Cr on the surface of the reinforcement phase can improve the interface characteristics of diamond, thereby enhancing its thermal conductivity.
2) Alloying treatment of the copper matrix. Before the composite processing of the material, pre-alloying treatment of metallic copper can produce composites with generally higher thermal conductivity.Doping active elements in the copper matrix can not only effectively reduce the wetting angle between diamond and copper but also generate a carbide layer that can dissolve in the copper matrix at the diamond/Cu interface after the reaction, thus modifying and filling most of the gaps present at the material interface, thereby improving thermal conductivity.
Research on thermal conductivity and thermal boundary improvement plays a decisive guiding role in the thermal management applications of high-power electronic devices.Measuring the boundary resistance of diamond substrates is also very sensitive. This means that the measurement may have a total of five unknown parameters: 1) thermal resistance at the aluminum film-diamond interface, 2) lateral thermal conductivity within the diamond, 3) longitudinal thermal conductivity within the diamond, 4) thermal capacity of the diamond, 5) thermal resistance at the diamond-substrate material interface. Even with certain analytical processing methods, as described in the equipment instructions (for details, please contact Shanghai Haoliang Optoelectronics), accurately extracting all unknown parameters is also very difficult.1)金刚石表面改性处理,例如在增强相表层镀Mo、Ti、W、Cr等活性元素可改善金刚石界面特性,从而提高其热传导性能。2)铜基体的合金化处理,在材料的复合加工之前,对金属铜进行预合金化处理,这样可制得热导率普遍较高的复合材料。在铜基体中掺杂活性元素不仅可有效降低金刚石与铜之间的润湿角,还能在反应后于金刚石/Cu界面间生成可固溶于铜基的碳化物层,这样材料界面间存在的多数间隙得到修饰填充,从而提高了导热性能。
热导率和热边界改善研究,使其对大功率电子器件的热管理应用决定性指导作用。
测量金刚石-基底边界电阻也很敏感。这意味着测量可能总共有五个未知参数:1)铝膜-金刚石间界面热阻2)金刚石内横向热导率3)金刚石内纵向热导率4)金刚石热容量5)金刚石-基底材料间界面热阻即使结合一定分析处理手段,见设备说明(详情联系请上海昊量光电),准确提取所有未知参数也很困难。
Previous:
Previous:
Recommended content
Share to